Cutiva™ PLUS Skin Closure System
from Resivant Medical

Flexible and Strong
The Cutiva™ PLUS Skin Closure System from Resivant Medical is the next generation of non-invasive skin closure system, combining the strength of super glue with the many benefits of silicone. Developed to be safe, secure and for a better user and patient experience.

At its core lies the Cross-linked Silicone Cyanoacrylate Adhesive, a breakthrough formula that ensures unparalleled strength and flexibility.
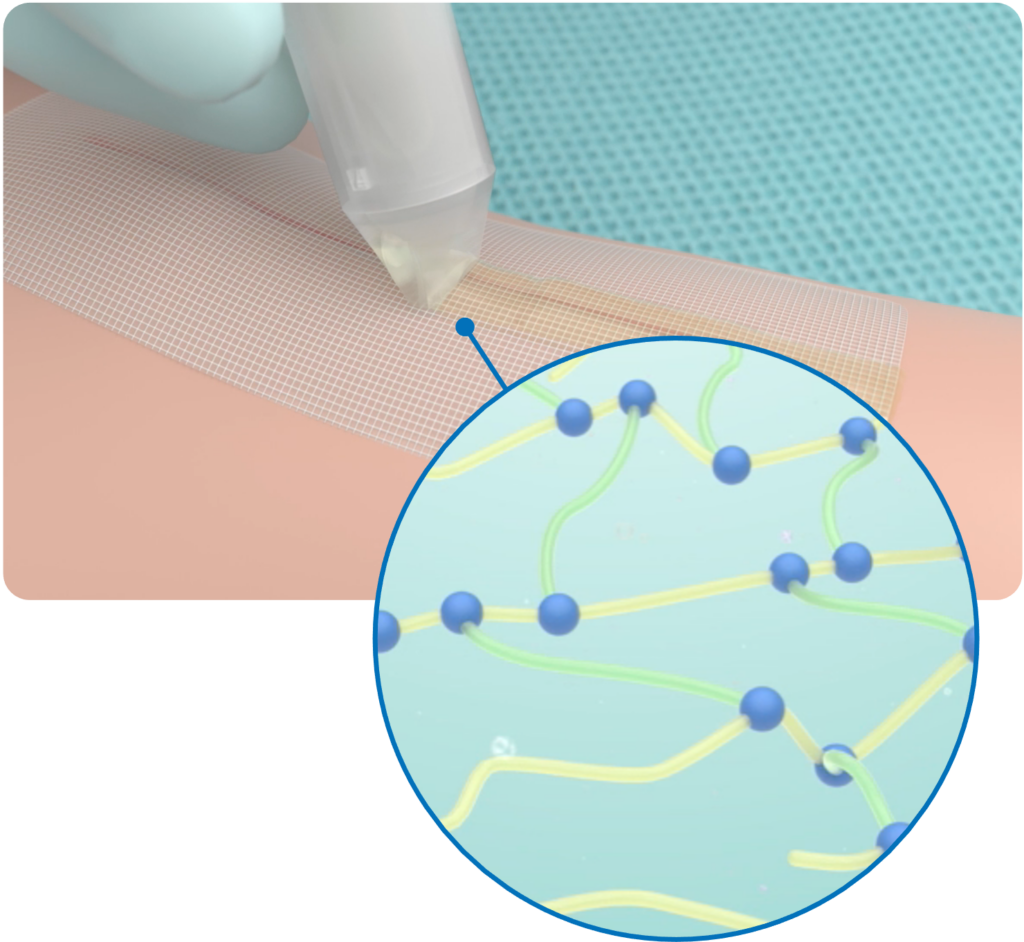
The Strength of Super Glue Crosslinked with the Many Benefits of Silicone
Crosslinking cyanoacrylate with silicone rubber in a skin closure system offers a versatile and effective solution for wound closure, providing strong adhesion, flexibility, moisture resistance, biocompatibility, and ease of application. These characteristics make it an ideal choice for a wide range of surgical wounds, helping to facilitate optimal wound healing and patient comfort.
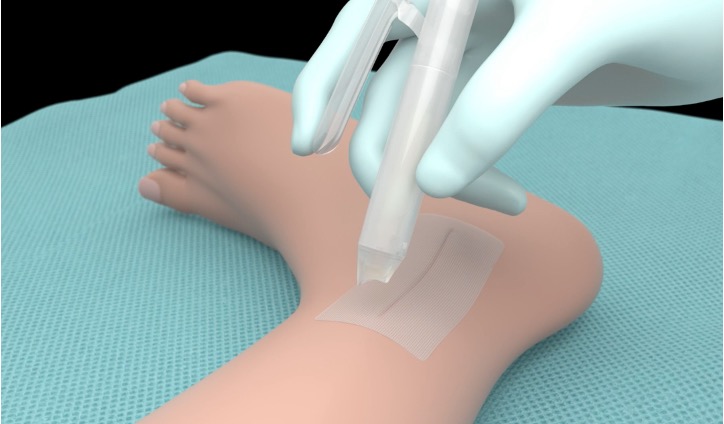
Designed for ease of use, the Cutiva™ PLUS system applicator enables one-handed setup and application, streamlining the closure process for healthcare professionals.
Ease of Use
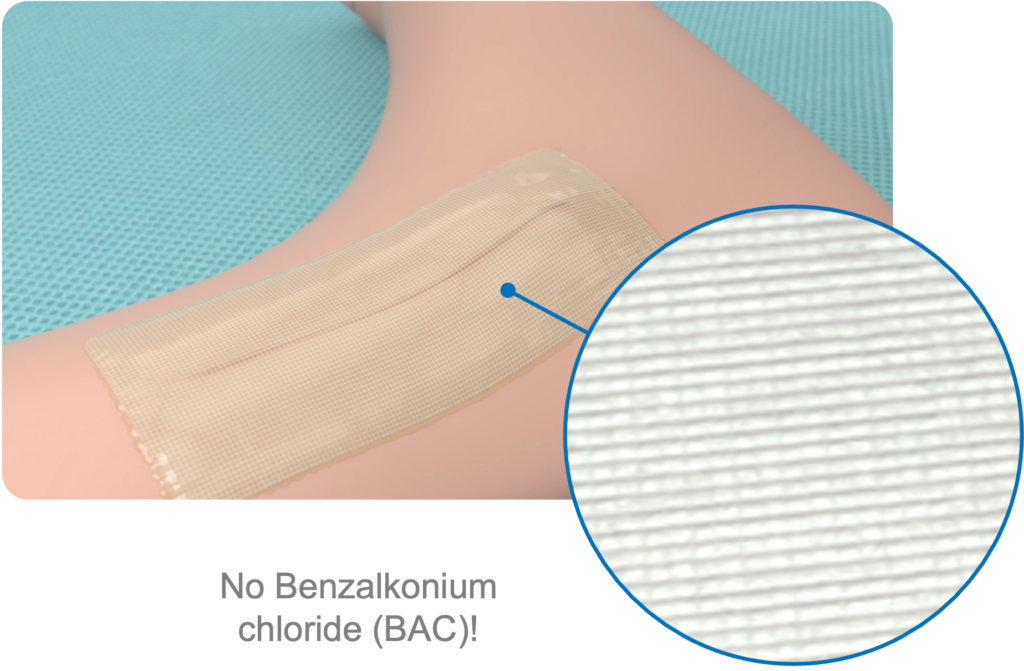
Enhancing its efficacy is the unique 10 cm self-adhering mesh patch that is first applied to the approximated skin edges until the liquid adhesive is applied to the mesh to achieve skin closure.
With a high viscosity self-setting no-run adhesive, application becomes effortless, allowing for precise placement and quick closure. The liquid adhesive and activator are mixed as they are dispensed from the applicator, meaning that 100% of the adhesive is activated.
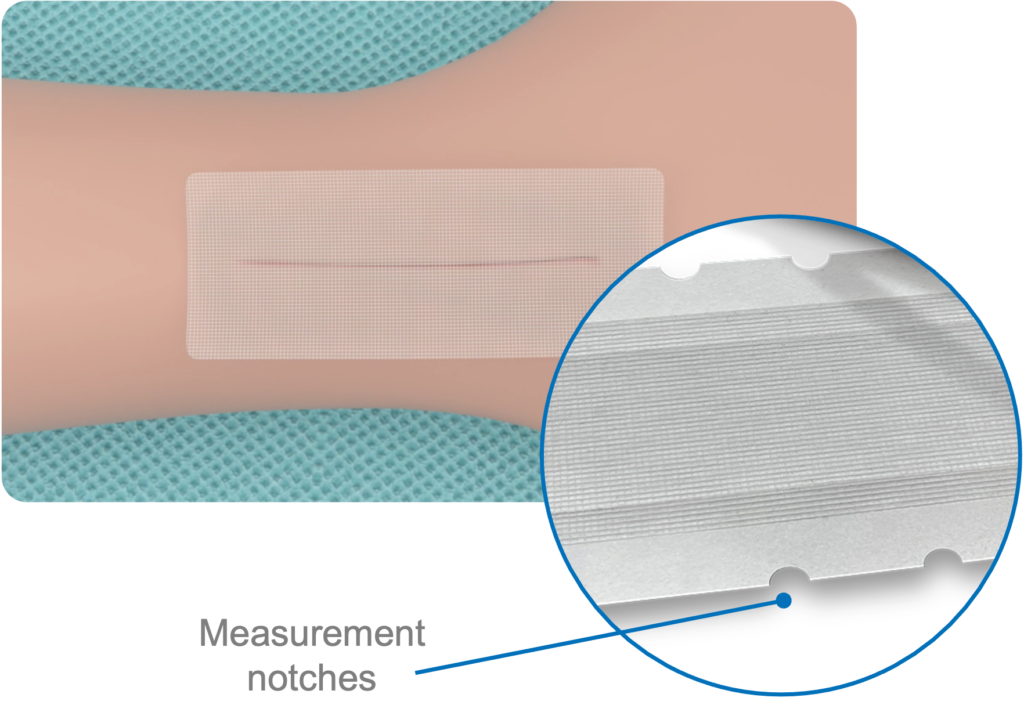
Mesh Patch
10cm Self-Adhering Polyester Mesh Patch, self-adhesive allows the mesh to be repositioned. The mesh patch also has notches to facilitate measurement of the wound and mesh to be applied.
Microbial Barrier
Cutiva™ PLUS Skin Closure System provides a physical barrier to microbial penetration. In vitro studies have been performed to demonstrate the microbial barrier properties of the Cutiva™ PLUS Skin Closure System for 72 hours after application.*
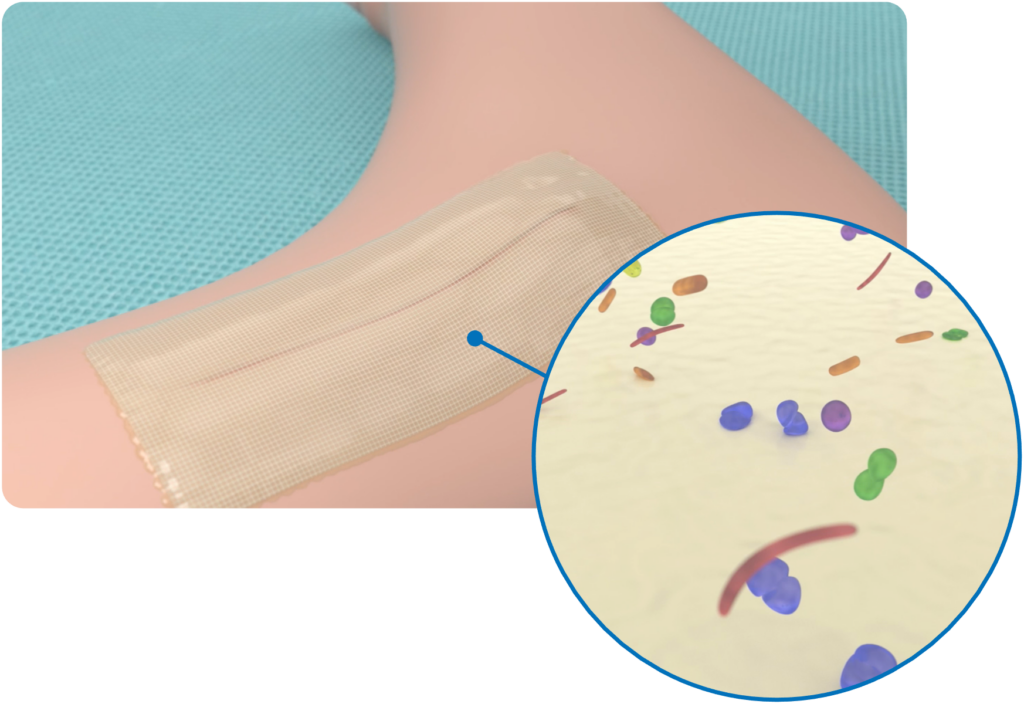
Safeguarding the Wound
Cutiva™ PLUS Skin Closure System’s advanced crosslinked formulation is designed to significantly reduce the risk of skin reactions, ensuring a comfortable experience for every patient.
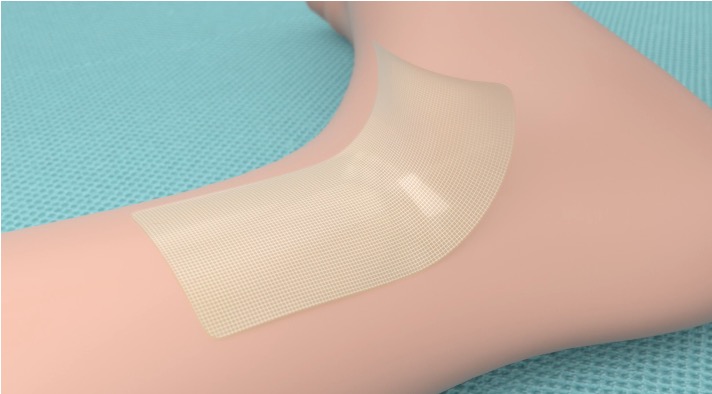
Transparent Film
Dries to a clear film, allowing easy monitoring of the wound site. No dressing required, often eliminates the need for an additional wound dressing.

Easy Painless Removal
Cutiva™ PLUS will need to remain in place for between 7 to 14 days until the wound incision is properly healed and is designed to be easily removed.
*No clinical studies have been performed and no clinical benefit associated with the in vitro microbial barrier performance of the device has been demonstrated.
